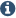Azaborines represent a class of molecules that bear a B–N bond in place of a C═C unit within an aromatic ring. These molecules possess aromaticity and stability similar but not identical to their corresponding all-carbon analogues. 2,1-Borazaronaphthalenes have received a significant amount of attention because of their potentially impactful properties in the fields of pharmaceuticals and materials science.
(1)
Having developed a general and convenient route for the construction of the 2,1-borazaronaphthalene core from 2-aminostyrene derivatives,
(2) we sought methods for the selective installation of various substituents about the assembled ring. For placement of substituents at the 3-position, the utilization of substituted alkenes of the styrene starting material failed to provide useful quantities of the desired target structures (eq
1).

Consequently, another approach, selective halogenation at the 3-position followed by substitution, was developed. The halogenation of 2,1-borazaronaphthalenes was first reported by Dewar in 1961 and was shown to be selective for reaction at the C-3 position, although yields were modest and the scope of the process was not extensively explored.
(3) More recent progress on the functionalization of 2,1-borazaronaphthalenes was achieved through modified conditions for the bromination, combined with subsequent Pd-catalyzed cross-coupling of 2,1-borazaronaphthalenes (Scheme
1).
(2)
This method allowed aryl and heteroaryl substrates to be successfully introduced to the ring system, but the path forward to install a variety of alkyl substituents at this position was unclear. Thus, although alkyl cross-coupling methods held some promise as a means to extend elaboration of the borazines at the 3-position, a primary goal was to demonstrate the incorporation of diverse alkyl substructures, and limitations on both the availability of the requisite organometallic starting materials and concerns about conversions in such cross-coupling reactions appeared daunting. Specifically, we sought the installation of saturated, heterocyclic ring systems, which are highly valued as moieties that possess nonplanar character, while allowing incorporation of hydrogen-bonding heteroatoms.
(4) Methods to insert these nonaromatic heterocycles onto aryl systems through typical sp
2–sp
3 cross-coupling methods have proven quite ineffective, yielding little to none of the desired products.
(5) Consequently, we pursued a more direct pathway for the efficient introduction of alkyl substituents onto the azaborine core.
Based on the stability of 2,1-borazaronaphthalenes to palladium catalysis, a metal-promoted reductive cross-coupling pathway
(6) for the introduction of alkyl halides onto 2,1-borazaronaphthalenes was envisioned. Weix et al.
(7) and Gong et al.
(8) have carried out extensive work in the area of reductive cross-couplings in the past decade, illustrating the ability to introduce alkyl halides to aromatic halides under Ni-catalyzed conditions. Recently, application of modified conditions based on these investigations that allow the reductive cross-coupling of nonaromatic heterocyclic bromides with aryl and heteroaryl bromides was reported (Scheme
2).
(9) We sought to extend a parallel coupling to 3-bromo-2,1-borazaronaphthalene systems, which we anticipated would react in a similar fashion with alkyl bromides.
Investigation into the reactivity of 3-bromo-2,1-borazaronaphthalenes under reductive coupling conditions was begun using 3-bromo-2-phenyl-2,1-borazaronaphthalene in the presence of
N-Boc-4-bromopiperidine. Under the conditions recently developed for the reductive coupling of nonaromatic heterocyclic bromides with aryl bromides,
(9) a 44% yield of the desired product was obtained (Scheme
3). The major side product observed was protodehalogenation of the azaborine.
Optimization on this system was begun by testing several bipyridine ligands, and the use of 4,4′-dimethyl-2,2′-bipyridine led to increased conversion to the desired product. Employing 1 equiv of 4-ethylpyridine (from 0.5 equiv) decreased the amount of observed protodehalogenation of the azaborine. Several inorganic salts were tested as additives (e.g., MgCl
2, CsI, LiCl, ZnBr
2, KBF
4, NaI), but NaBF
4 led to markedly cleaner reactions than the other salts explored. The use of
N-Boc-4-iodopiperidine in place of the corresponding bromide allowed the reaction to be carried out at 40 °C, and the formation of side products was significantly diminished. Various polar solvents were tested under the reaction conditions, and it was determined that both MeOH and DMA served as good reaction solvents in combination with nonpolar cosolvents. The best solvent combinations were mixtures of either DMA or MeOH with cyclohexane, and variation of the ratios of these solvent combinations showed that a 2:1 cyclohexane/DMA mixture was optimal (Table
1). Reduction of the amount of alkyl iodide to 1 equiv (from 1.2 equiv) was detrimental to the reaction, as was lowering the temperature below 40 °C. The use of anhydrous, degassed solvents was determined to be unnecessary, allowing the coupling to be conducted in solvents that did not require degassing or drying prior to use. Lowering the catalyst loading from 10 to 5 mol % Ni did not decrease the conversion to any significant extent.
With optimized conditions in hand, the reductive cross-coupling of
N-Boc-4-iodopiperidine was carried out with a variety of 3-bromo-2,1-borazaronaphthalenes (Table
2). The reaction conditions were amenable to the incorporation of various aromatic moieties on boron, including fluoro-, methoxy-, and carbomethoxy-substituted phenyl rings (entries 2–5). Sterically hindered
B-heterocyclic 2,1-borazaronaphthalenes were successfully engaged in the reaction, such that the dibenzofuran and dibenzothiophene systems afforded the cross-coupled products in high yield (entries 6 and 7). Alkyl substituents on boron were also tolerated under the reaction conditions (entries 8–10). We were delighted to see that
N-substituted systems reacted under the developed conditions (entries 11–13). An allyl substitution on nitrogen is not cleaved during the coupling, demonstrating the inherent aromaticity and stability of 2,1-borazaronaphthalenes.
The developed method was next extended to the cross-coupling of 3-bromo-2-phenyl-2,1-borazaronaphthalene with a variety of alkyl iodides (Table
3). Other nonaromatic heterocyclic iodides were successfully cross-coupled, including azetidine, tetrahydropyran, tetrahydrofuran, and oxetane systems (entries 1–5). The scalable nature of the couplings was demonstrated by carrying out the reaction of 3-bromo-2-phenyl-2,1-borazaronaphthalene with
N-Boc-3-iodoazetidine on a 3.0 mmol scale (2.5 mol % of Ni, decreased from 5 mol % of Ni on a 0.5 mmol scale) without a decrease in yield (entry 2). The method was further extended to show application to non-heteroatom-containing alkyl iodides, and both secondary and primary alkyl iodides were readily cross-coupled (entries 6–8). Attempts to employ tertiary alkyl iodides or sterically hindered secondary alkyl iodides (e.g., 2-iodoadamantane) resulted in low conversion to product (less than 20%).
To demonstrate the applicability of this method toward alkylation of azaborine cores at another position,
N-Boc-4-iodopiperidine was cross-coupled with 6-bromo-2-methyl-3-phenyl-2,1-borazaronaphthalene (eq
2). The reaction proceeded in 51% yield without any change in the developed conditions, illustrating the potential for this method to be extended beyond 3-bromo-2,1-borazaronaphthalene systems.

In conclusion, a method has been developed for the reductive cross-coupling of 3-bromo-2,1-borazaronaphthalenes with alkyl iodides. The method allows the coupling of azaborine cores bearing aryl or alkyl substituents on boron and also tolerates alkyl substitution on nitrogen. Primary and secondary alkyl iodides, including an important class of nonaromatic heterocycles, are capable of reacting under the developed conditions. This method provides a valuable, direct route to alkylated, functionalized azaborine systems.
Complete experimental procedures and characterization data (
1H,
13C,
11B NMR, IR, mp, HRMS). This material is available free of charge via the Internet at
http://pubs.acs.org.
†
Author Contributions
These authors contributed equally.
The authors declare no competing financial interest.
Acknowledgment
This research was supported by the NIGMS (R01 GM-081376) and Eli Lilly. Frontier Scientific is acknowledged for their generous donation of potassium organotrifluoroborates. Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for acquisition of HRMS spectra.
References
This article references 9 other publications.
1.
(a)
Liu, Z.;
Marder, T. B. Angew. Chem., Int. Ed. 2008,
47,
242
[
Crossref], [
PubMed], [
CAS]
1.
B-N versus C-C: how similar are they?
Liu, Zhiqiang; Marder, Todd B.
Angewandte Chemie, International Edition (2008), 47 (2), 242-244CODEN: ACIEF5; ISSN:1433-7851. (Wiley-VCH Verlag GmbH & Co. KGaA)
Preliminary DFT calcns. on I are reported and discussed in terms of bond localization. Preceding this is a review of cyclic hydrocarbons in which B-N units replace their isoelectronic C-C counterparts. The recent report of Piers et al. (2007) on the synthesis, crystal structure, and optical properties of 10a-aza-10b-borapyrene represents a major breakthrough in this field.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD1cXht1Smsb4%253D&md5=8ad12001eca14bc72af3e1a87c140992
(b)
Bosdet, M. J. D.;
Piers, W. E. Can. J. Chem. 2009,
87,
8
[
Crossref], [
CAS]
1.
B-N as a C-C substitute in aromatic systems
Piers, Warren E.; Bosdet, Michael J. D.
Canadian Journal of Chemistry (2009), 87 (1), 8-29CODEN: CJCHAG; ISSN:0008-4042. (National Research Council of Canada)
A review with 184 refs. on synthesis and electronic structure of B-N-heteroarom. 6- and 5-membered compds. in which substitution of isoelectronic B-N units for C:C units in arom. hydrocarbons produces novel heterocycles with structural similarities to the all-carbon frameworks, but with fundamentally altered electronic properties and chem. Condensed and heterocyclic systems are also included; brief summary of B-N-substitution in fullerenes and carbon nanotubes is presented. Since the pioneering work of Dewar some 50 years ago, the relationship between B-N and C-C and the wealth of parent all-carbon aroms. has captured the imagination of org., inorg., materials, and computational chemists alike, particularly in recent years. New applications in biol. chem., new materials, and novel ligands for transition-metal complexes have emerged from these studies. Structural aspects pertaining to the retention of aromaticity are emphasized, along with delineation of significant differences in phys. properties of the B-N compd. as compared to the C:C parent.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD1cXhsFagsbjK&md5=06f4a9a264f258f8e673a33d1154bd9c
(c)
Campbell, P. G.;
Marwitz, A. J. V.;
Liu, S.-Y. Angew. Chem., Int. Ed. 2012,
51,
6074
[
Crossref], [
PubMed], [
CAS]
1.
Recent Advances in Azaborine Chemistry
Campbell, Patrick G.; Marwitz, Adam J. V.; Liu, Shih-Yuan
Angewandte Chemie, International Edition (2012), 51 (25), 6074-6092CODEN: ACIEF5; ISSN:1433-7851. (Wiley-VCH Verlag GmbH & Co. KGaA)
A review. The chem. of organoboron compds. has been primarily dominated by their use as powerful reagents in synthetic org. chem. Recently, the incorporation of boron as part of a functional target structure has emerged as a useful way to generate diversity in org. compds. A commonly applied strategy is the replacement of a CC unit with its isoelectronic BN unit. In particular, the BN/CC isosterism of the ubiquitous arene motif has undergone a renaissance in the past decade. The parent mol. of the 1,2-dihydro-1,2-azaborine family has now been isolated. New mono- and polycyclic B,N heterocycles have been synthesized for potential use in biomedical and materials science applications. This review is a tribute to Dewar's first synthesis of a monocyclic 1,2-dihydro-1,2-azaborine 50 years ago and discusses recent advances in the synthesis and characterization of heterocycles that contain carbon, boron, and nitrogen.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC38Xns1Wktb0%253D&md5=82e79d7a5dc99d88d3103ba2f9a4173f
(d)
Marwitz, A. J. V.;
Matus, M. H.;
Zakharov, L. N.;
Dixon, D. A.;
Liu, S.-Y. Angew. Chem., Int. Ed. 2009,
48,
973
[
Crossref], [
PubMed], [
CAS]
1.
A hybrid organic/inorganic benzene
Marwitz, Adam J. V.; Hatus, Myrna H.; Zakharov, Lev N.; Dixon, David A.; Liu, Shih-Yuan
Angewandte Chemie, International Edition (2009), 48 (5), 973-977CODEN: ACIEF5; ISSN:1433-7851. (Wiley-VCH Verlag GmbH & Co. KGaA)
1,2-Dihydro-1,2-azaborine, a hybrid org./inorg. benzene, is a stable arom. mol. with features that are distinct from its isoelectronic "org." (benzene) and "inorg." (borazine) counterparts. Exptl. structural, spectroscopic, and chem. data are fully supported by high-level calcns.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD1MXhs1yktLY%253D&md5=8e83f0249dae6dfe87cc5bb7f394fe6d
(e)
Campbell, P. G.;
Abbey, E. R.;
Neined, D.;
Grant, D. J.;
Dixon, D. A.;
Liu, S.-Y. J. Am. Chem. Soc. 2010,
132,
18048
[
ACS Full Text 
], [
CAS]
1.
Resonance Stabilization Energy of 1,2-Azaborines: A Quantitative Experimental Study by Reaction Calorimetry
Campbell, Patrick G.; Abbey, Eric R.; Neiner, Doinita; Grant, Daniel J.; Dixon, David A.; Liu, Shih-Yuan
Journal of the American Chemical Society (2010), 132 (51), 18048-18050CODEN: JACSAT; ISSN:0002-7863. (American Chemical Society)
The authors provided the 1st exptl. detn. of the resonance stabilization energy of dihydro- and tetrahydro-1,2-azaborines through hydrogenation enthalpy measurements. Arom. and single-olefin six-membered BN heterocycles were synthesized, and the heats of hydrogenation were measured calorimetrically. A comparison of the hydrogenation enthalpies of these compds. revealed that 1,2-azaborines have a resonance stabilization energy of 16.6 ± 1.3 kcal/mol, in good agreement with calcd. values.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3cXhsFCltLjE&md5=d8ca0c5a7e618d45c28b47397c9bd93e
(f)
Abbey, E. R.;
Zakharov, L. N.;
Liu, S.-Y. J. Am. Chem. Soc. 2008,
130,
7520
[
ACS Full Text 
]
There is no corresponding record for this reference.
2.
Molander, G. A.; Wisniewski, S. R. Manuscript submitted.
There is no corresponding record for this reference.
3.
Dewar, M. J. S.;
Dietz, R. J. Org. Chem. 1961,
26,
3253
[
ACS Full Text 
], [
CAS]
3.
New heteroaromatic compounds. XV. Halogenation of 2-methyl-2,1-borazaronaphthalene
Dewar, Michael J. S.; Dietz, Roy
Journal of Organic Chemistry (1961), 26 (), 3253-6CODEN: JOCEAH; ISSN:0022-3263.
cf. CA 55, 25977a; preceeding abstr. Bromination of 2-methyl-2,1-borazaronaphthalene (I) (Dewar, et al., CA 55, 19940e.) gave a mixt. of the 3-Br deriv. (II) of I and o-H2NC6H4CH:CHZ (III) (Z = Br); chlorination gave analogous products. The mechanism of halogenation was discussed. 2-O2NC6H4CH:CHCO2H treated with HOCl gave 7% 2-O2NC6H4CH:CHCl (IV), m. 55.5-6.5°. Redn. of IV with SnCl2 and aq. alc. HCl gave 61% III (Z = Cl), m. 54-5.5°. III (Z = Cl) (1.16 g.) in 10 ml. CH2Cl2 added slowly to 2 g. BCl3 at -70°, the mixt. allowed to warm to room temp. (no HCl evolved), concd. at 0.5 mm. at 20°, the residual oil dissolved in 30 ml. dry C6H6, the soln. heated, boiled 4 hrs. while sweeping with dry N (1.5 millimoles HCl absorbed in H2O; no H3BO3 detected by further titration in the presence of mannitol), cooled, treated with 50 ml. Et2O and 20 ml. H2O, the org. layer washed with 2N HCl, dried, and evapd. gave 1.26 g. bis(3-chloro-2,1-borazaro-2-naphthyl)ether (V), m. 240-1.5° (C6H6-petr. ether). Crystn. of V from MeOH gave 2-methoxy-3-chloro-2,1-borazaronaphthalene, m. 88-9.5°, slowly hydrolyzing quant. to V on storage in air. V (0.5 g.) slurried in 40 ml. Et2O, added to 7.1 millimoles MeMgI in 30 ml. Et2O with stirring at room temp., the mixt. refluxed 3 hrs., hydrolyzed with 30 ml. 2N HCl, the Et2O layer dried, evapd., the residual solid (0.52 g.) chromatographed on Al2O3, and eluted with petr. ether gave 0.48 g. 3-Cl deriv. (VI) of I, m. 119-20.5° (petr. ether). Cl (15 millimoles) in AcOH added during 1 hr. to 2.01 g. I in 50 ml. AcOH with stirring at 18°, stirred 1 hr., treated with 800 ml. H2O, the ppt. filtered off, dissolved in 80 ml. Et2O, the soln. washed with H2O, 2N HCl, and H2O, dried, evapd., the residue (0.92 g.) chromatographed on Al2O3, and eluted with 50 ml. petr. ether (0.05 g. I recovered) then with 7 l. petr. ether gave 0.62 g. VI, m. 119-20.5°; the combined aq. filtrate and washings made alk. with aq. NH3 (d. 0.880), the product (1.46 g.) isolated with Et2O, and extd. with boiling pentane gave 1.41 g. III (Z = Cl), m. 50.5-2.0° (pentane). Similarly, bromination of I gave II, m. 128-9.5°, and III (Z = Br), m. 78.5-9.5°. Cl (15 millimoles) in CCl4 (0.5M) added during 1 hr. to 1.82 g. I in 50 ml. CCl4, the soln. stirred 1 hr., washed with 70 ml. H2O, 50 ml. 2N HCl, and 50 ml. H2O, dried, evapd., the residual tarry solid digested with Et2O, the ext. evapd., and the solid (0.24 g.) chromatographed gave 0.17 g. VI, m. 117.5-19.5°; the combined aq. washings made strongly alk. with aq. NH3 and the product isolated with Et2O gave 1.20 g. III (Z = Cl), m. 52.5-4.0°. The effect of a slight variation in conditions on the halogenation of I were summarized. The ultraviolet spectra of I, II, VI, III (Z = H), III (Z = Br), and III (R = Cl) were recorded.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADyaF38Xms12kug%253D%253D&md5=a636fdf67e87c2ac2ad6ddd377c2663a
4.
Loverling, F.;
Bikker, J.;
Humblet, C. J. Med. Chem. 2009,
52,
6752
[
ACS Full Text 
]
There is no corresponding record for this reference.
5.
Allwood, D. M.;
Blakemore, D. C.;
Brown, A. D.;
Ley, S. V. J. Org. Chem. 2014,
79,
328
[
ACS Full Text 
], [
CAS]
5.
Metal-Free Coupling of Saturated Heterocyclic Sulfonylhydrazones with Boronic Acids
Allwood, Daniel M.; Blakemore, David C.; Brown, Alan D.; Ley, Steven V.
Journal of Organic Chemistry (2014), 79 (1), 328-338CODEN: JOCEAH; ISSN:0022-3263. (American Chemical Society)
The coupling of arom. moieties with satd. heterocyclic partners is currently an area of significant interest for the pharmaceutical industry. Herein, we present a procedure for the metal-free coupling of 4-, 5-, and 6-membered satd. heterocyclic p-methoxyphenyl (PMP) sulfonylhydrazones with aryl and heteroarom. boronic acids. This procedure enables a simple, two-step synthesis of a range of functionalized sp2-sp3 linked bicyclic building blocks, including oxetanes, piperidines, and azetidines, from their parent ketones.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3sXhvFWlt77P&md5=36b8079121ce6cdfee8c2ba46b6b5297
6.
For examples of reductive cross-coupling reactions, see:
(a)
Durandetti, M.;
Nédélec, J.-Y.;
Périchon, J. J. Org. Chem. 1996,
61,
1748
[
ACS Full Text 
], [
CAS]
6.
Nickel-Catalyzed Direct Electrochemical Cross-Coupling between Aryl Halides and Activated Alkyl Halides
Durandetti, Muriel; Nedelec, Jean-Yves; Perichon, Jacques
Journal of Organic Chemistry (1996), 61 (5), 1748-55CODEN: JOCEAH; ISSN:0022-3263. (American Chemical Society)
The electrochem. redn. of a mixt. of aryl halides and activated alkyl halides in DMF in the presence of catalytic amt. of NiBr2bipy leads to cross-coupling products in good to high yields. The method applies to the synthesis of α-aryl ketones, α-aryl esters, and allylated compds. from readily available org. halides. Optimization of the process has been obtained by slowly adding the most reactive org. halide (usually the activated alkyl halide) during the electrolysis which is best conducted at 70 °C when aryl bromides are involved.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADyaK28XhtFersLc%253D&md5=16fab4777bb5c8abcbc7377e67ca97bf
(b)
Durandetti, M.;
Gosmini, C.;
Périchon, J. Tetrahedron 2007,
63,
1146
[
Crossref], [
CAS]
6.
Ni-catalyzed activation of α-chloroesters: a simple method for the synthesis of α-arylesters and β-hydroxyesters
Durandetti, Muriel; Gosmini, Corinne; Perichon, Jacques
Tetrahedron (2006), 63 (5), 1146-1153CODEN: TETRAB; ISSN:0040-4020. (Elsevier Ltd.)
Coupling reactions of α-chloro esters with aryl halides (α-arylation) or carbonyl compds. (Reformatsky) using nickel catalyst allowed, under mild conditions, the prepn. of various functionalized aryl propionic acid derivs. or β-hydroxy esters. In the synthesis of aryl propionic acid derivs., the process was efficient with aryl halides bearing either electron-withdrawing or electron-donating groups.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD2sXis1Wn&md5=e5ac55427e7bcabfcf832322756b4829
(c)
Gosmini, C.;
Bassene-Ernst, C.;
Durandetti, M. Tetrahedron 2009,
65,
6141
[
Crossref], [
CAS]
6.
Synthesis of functionalized 2-arylpyridines from 2-halopyridines and various aryl halides via a nickel catalysis
Gosmini, Corinne; Bassene-Ernst, Carine; Durandetti, Muriel
Tetrahedron (2009), 65 (31), 6141-6146CODEN: TETRAB; ISSN:0040-4020. (Elsevier Ltd.)
An efficient nickel-catalyzed method devoted to the direct formation of functionalized 2-arylpyridines is described avoiding the prior prepn. of organometallic species. Various functionalized 2-arylpyridines are obtained in moderate to excellent yields by a one-step chem. procedure from corresponding halides. The NiBr2(2,2'-bipyridine) complex appears to be an extremely suitable catalyst for the activation in the presence of manganese dust of arom. halides and pyridyl halides functionalized by reactive groups. The versatility of this original process represents a simple alternative to most known methods using organometallic reagents.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD1MXotFCktro%253D&md5=ac4478baba4509b8162cd7ff1dc9aca6
(d)
Yan, C.-S.;
Peng, Y.;
Xu, X.-B.;
Wang, Y.-W. Chem.—Eur. J. 2012,
18,
6039
[
Crossref], [
PubMed], [
CAS]
6.
Nickel-Mediated Inter- and Intramolecular Reductive Cross-Coupling of Unactivated Alkyl Bromides and Aryl Iodides at Room Temperature
Yan, Chang-Song; Peng, Yu; Xu, Xiao-Bo; Wang, Ya-Wen
Chemistry--A European Journal (2012), 18 (19), 6039-6048, S6039/1-S6039/224CODEN: CEUJED; ISSN:0947-6539. (Wiley-VCH Verlag GmbH & Co. KGaA)
A nickel-mediated intermol. reductive cross-coupling reaction of unactivated alkyl bromides and aryl iodides at room temp. has been developed and successfully extended to less explored intramol. versions and tandem cyclization-intermol. cross-coupling. Highly stereoselective (or stereospecific) synthesis of linear-fused perhydrofuro[2,3-b]furan (pyran) and spiroketal skeletons allows rapid access to these useful building blocks, which would be potentially valuable in the synthesis of relevant natural products. A rational explanation for the formation of contiguous stereogenic centers is given.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC38XkvVKit7o%253D&md5=ccfd5a7aa8643d076dc079e1961af443
(e)
Gomes, P.;
Gosmini, C.;
Perichon, J. Org. Lett. 2003,
5,
1043
[
ACS Full Text 
], [
CAS]
6.
New Chemical Cross-Coupling between Aryl Halides and Allylic Acetates Using a Cobalt Catalyst
Gomes, Paulo; Gosmini, Corinne; Perichon, Jacques
Organic Letters (2003), 5 (7), 1043-1045CODEN: ORLEF7; ISSN:1523-7060. (American Chemical Society)
The cobalt-catalyzed coupling reaction of arom. halides and allyl acetate proceeds readily under mild conditions in the presence of the appropriate reducing agent to produce allylbenzenes, e.g., I, either in pure acetonitrile (aryl bromides) or in an acetonitrile/pyridine mixt. (aryl chlorides).
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD3sXhslynsbo%253D&md5=f6b665230eea2ce188baf8a04dcd8cc8
(f)
Cherney, A. H.;
Kadunce, N. T.;
Reisman, S. E. J. Am. Chem. Soc. 2013,
135,
7442
[
ACS Full Text 
], [
CAS]
6.
Catalytic Asymmetric Reductive Acyl Cross-Coupling: Synthesis of Enantioenriched Acyclic α,α-Disubstituted Ketones
Cherney, Alan H.; Kadunce, Nathaniel T.; Reisman, Sarah E.
Journal of the American Chemical Society (2013), 135 (20), 7442-7445CODEN: JACSAT; ISSN:0002-7863. (American Chemical Society)
The first enantioselective Ni-catalyzed reductive acyl cross-coupling has been developed. Treatment of acid chlorides and racemic secondary benzyl chlorides with a NiII/bis(oxazoline) catalyst in the presence of Mn0 as a stoichiometric reductant generates acyclic α,α-disubstituted ketones in good yields and high enantioselectivity without requiring stoichiometric chiral auxiliaries or pregeneration of organometallic reagents. The mild, base-free reaction conditions are tolerant of a variety of functional groups on both coupling partners.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3sXmvVyhuro%253D&md5=19300c6d0f77d3f49a85ee4c5c7d9de6
(g)
Leon, T.;
Correa, A.;
Martin, R. J. Am. Chem. Soc. 2013,
135,
1221
[
ACS Full Text 
], [
CAS]
6.
Ni-Catalyzed Direct Carboxylation of Benzyl Halides with CO2
Leon, Thierry; Correa, Arkaitz; Martin, Ruben
Journal of the American Chemical Society (2013), 135 (4), 1221-1224CODEN: JACSAT; ISSN:0002-7863. (American Chemical Society)
A novel Ni-catalyzed carboxylation of benzyl halides with CO2 has been developed. The described carboxylation reaction proceeds under mild conditions (atm. CO2 pressure) at room temp. Unlike other routes for similar means, this method does not require well-defined and sensitive organometallic reagents and thus is a user-friendly and operationally simple protocol for assembling phenylacetic acids.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3sXms1OgtQ%253D%253D&md5=9b282fc5c6dc6c11abc6e92175e5d20f
(h)
Knappke, C. E. I.;
Grupe, S.;
Gartner, D.;
Corpet, M.;
Gosmini, C.;
Jacobi von Wangelin, A. Chem.—Eur. J. 2014,
20,
6828
[
Crossref], [
PubMed], [
CAS]
6.
Reductive cross-coupling reactions between two electrophiles
Knappke, Christiane E. I.; Grupe, Sabine; Gaertner, Dominik; Corpet, Martin; Gosmini, Corinne; Jacobi von Wangelin, Axel
Chemistry - A European Journal (2014), 20 (23), 6828-6842CODEN: CEUJED; ISSN:0947-6539. (Wiley-VCH Verlag GmbH & Co. KGaA)
A review. Reductive cross-electrophile coupling reactions were recently developed to a versatile and sustainable synthetic tool for selective C-C bond formation. The employment of cheap and abundant electrophiles avoids the pre-formation and handling of organometallic reagents. In situ reductive coupling is effected in the presence of a transition-metal catalyst (Ni, Co, Pd, Fe) and a suitable metallic reductant (Mn, Zn, Mg). This concept article assessed the current state of the art and summarized recent protocols with various combinations of alkyl, alkenyl, allyl, and aryl reagents and highlights key mechanistic studies.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2cXnvFSksb8%253D&md5=6294ddb030bd43a85f7e330756640c21
7.
(a)
Everson, D. A.;
Shrestha, R.;
Weix, D. J. J. Am. Chem. Soc. 2010,
132,
920
[
ACS Full Text 
], [
CAS]
7.
Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides
Everson, Daniel A.; Shrestha, Ruja; Weix, Daniel J.
Journal of the American Chemical Society (2010), 132 (3), 920-921CODEN: JACSAT; ISSN:0002-7863. (American Chemical Society)
The direct reductive cross-coupling of alkyl halides with aryl halides is described. The transformation is efficient (equimolar amts. of the starting materials are used), generally high-yielding (all but one between 55 and 88% yield), highly functional-group-tolerant [OH, NHBoc, NHCbz, Bpin, C(O)Me, CO2Et, and CN are all tolerated], and easy to perform (uses only benchtop-stable reagents, tolerates small amts. of water and oxygen, changes color when complete, and uses filtration workup). The reaction appears to avoid the formation of intermediate organomanganese species, and a synergistic effect was found when a mixt. of two ligands was employed.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3cXhtFCqug%253D%253D&md5=dc1a9ba89588d8bba926ecba632c132d
(b)
Everson, D. A.;
Jones, B. A.;
Weix, D. J. J. Am. Chem. Soc. 2012,
134,
6146
[
ACS Full Text 
], [
CAS]
7.
Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides
Everson, Daniel A.; Jones, Brittany A.; Weix, Daniel J.
Journal of the American Chemical Society (2012), 134 (14), 6146-6159CODEN: JACSAT; ISSN:0002-7863. (American Chemical Society)
A general method is presented for the synthesis of alkylated arenes by the chemoselective combination of two electrophilic carbons. Under the optimized conditions, a variety of aryl and vinyl bromides are reductively coupled with alkyl bromides in high yields. Under similar conditions, activated aryl chlorides can also be coupled with bromoalkanes. The protocols are highly functional-group tolerant (-OH, -NHTs, -OAc, -OTs, -OTf, -COMe, -NHBoc, -NHCbz, -CN, -SO2Me), and the reactions are assembled on the benchtop with no special precautions to exclude air or moisture. The reaction displays different chemoselectivity than conventional cross-coupling reactions, such as the Suzuki-Miyaura, Stille, and Hiyama-Denmark reactions. Substrates bearing both an electrophilic and nucleophilic carbon result in selective coupling at the electrophilic carbon (R-X) and no reaction at the nucleophilic carbon (R-[M]) for organoboron (-Bpin), organotin (-SnMe3), and organosilicon (-SiMe2OH) contg. org. halides (X-R-[M]). A Hammett study showed a linear correlation of σ and σ(-) parameters with the relative rate of reaction of substituted aryl bromides with bromoalkanes. The small ρ values for these correlations (1.2-1.7) indicate that oxidative addn. of the bromoarene is not the turnover-frequency detg. step. The rate of reaction has a pos. dependence on the concn. of alkyl bromide and catalyst, no dependence upon the amt. of zinc (reducing agent), and an inverse dependence upon aryl halide concn. These results and studies with an org. reductant (TDAE) argue against the intermediacy of organozinc reagents.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC38XkvVent7s%253D&md5=0e68c86b49a0a21d05c806a789aa9be0
(c)
Biswas, S.;
Weix, D. J. J. Am. Chem. Soc. 2013,
135,
16192
[
ACS Full Text 
], [
CAS]
7.
Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides
Biswas, Soumik; Weix, Daniel J.
Journal of the American Chemical Society (2013), 135 (43), 16192-16197CODEN: JACSAT; ISSN:0002-7863. (American Chemical Society)
The direct cross-coupling of two different electrophiles, such as an aryl halide with an alkyl halide, offers many advantages over conventional cross-coupling methods that require a carbon nucleophile. Despite its promise as a versatile synthetic strategy, a limited understanding of the mechanism and origin of cross selectivity has hindered progress in reaction development and design. Herein, we shed light on the mechanism for the nickel-catalyzed cross-electrophile coupling of aryl halides with alkyl halides and demonstrate that the selectivity arises from an unusual catalytic cycle that combines both polar and radical steps to form the new C-C bond.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3sXhtlSjtbfN&md5=f5874e0a954d7274d21a41f7ef77bf85
(d)
Everson, D. A.;
Buonomo, J. A.;
Weix, D. J. Synlett 2014,
14,
3352
There is no corresponding record for this reference.
(e)
Everson, D. A.;
Weix, D. J. J. Org. Chem. 2014,
79,
4793
[
ACS Full Text 
], [
CAS]
7.
Cross-Electrophile Coupling: Principles of Reactivity and Selectivity
Everson, Daniel A.; Weix, Daniel J.
Journal of Organic Chemistry (2014), 79 (11), 4793-4798CODEN: JOCEAH; ISSN:0022-3263. (American Chemical Society)
A review. A crit. overview of the catalytic joining of two different electrophiles, cross-electrophile coupling (XEC), is presented with an emphasis on the central challenge of cross-selectivity. Recent synthetic advances and mechanistic studies have shed light on four possible methods for overcoming this challenge: (1) employing an excess of one reagent; (2) electronic differentiation of starting materials; (3) catalyst-substrate steric matching; and (4) radical chain processes. Each method is described using examples from the recent literature.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2cXnvVSnt7o%253D&md5=2c8ee4bbc060e18550fb8fc9a3e2af10
8.
(a)
Yu, X.;
Yang, T.;
Wang, S.;
Xu, H.;
Gong, H. Org. Lett. 2011,
13,
2138
[
ACS Full Text 
], [
CAS]
8.
Nickel-Catalyzed Reductive Cross-Coupling of Unactivated Alkyl Halides
Yu, Xiaolong; Yang, Tao; Wang, Shulin; Xu, Hailiang; Gong, Hegui
Organic Letters (2011), 13 (8), 2138-2141CODEN: ORLEF7; ISSN:1523-7052. (American Chemical Society)
A Ni-catalyzed reductive approach to the cross-coupling of two unactivated alkyl halides has been successfully developed. The reaction works efficiently for primary and secondary halides, with at least one being bromide. The mild reaction conditions allow for excellent functional group tolerance and provide the C(sp3)-C(sp3) coupling products in moderate to excellent yields.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3MXjsleqsb4%253D&md5=125b9741c20d17627adb3d2f2594774c
(b)
Xu, H.;
Zhao, C.;
Qian, Q.;
Deng, W.;
Gong, H. Chem. Sci. 2013,
4,
4022
[
Crossref], [
CAS]
8.
Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant
Xu, Hailiang; Zhao, Chenglong; Qian, Qun; Deng, Wei; Gong, Hegui
Chemical Science (2013), 4 (10), 4022-4029CODEN: CSHCCN; ISSN:2041-6520. (Royal Society of Chemistry)
(Pinacolato)diboron was used as the terminal reductant which allowed the efficient Ni-catalyzed coupling of unactivated secondary and primary alkyl halides, generating the C(sp3)-C(sp3) coupling products in good yields. The mild catalytic conditions displayed an excellent functional group tolerance and good chemoselectivities which required only 1.5 equiv. of primary bromides for the coupling with secondary bromides. Mechanistic studies suggest that an in-situ organoborane/Suzuki process was not likely and was proved that the base and ligand had more profound impact on selecting this reductive coupling pathway. The good chemoselectivity appears to be evoked by the formation of Ni-Bpin catalytic intermediates which demands matched sizes and reactivities of the alkyl halide coupling partners for optimal coupling efficiency.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3sXhtlent77J&md5=b3a6e9e570f1dfb92e7570010d0f03b6
(c)
Wang, S.;
Qian, Q.;
Gong, H. Org. Lett. 2012,
14,
3352
[
ACS Full Text 
], [
CAS]
8.
Nickel-Catalyzed Reductive Coupling of Aryl Halides with Secondary Alkyl Bromides and Allylic Acetate
Wang, Shulin; Qian, Qun; Gong, Hegui
Organic Letters (2012), 14 (13), 3352-3355CODEN: ORLEF7; ISSN:1523-7052. (American Chemical Society)
A room-temp. Ni-catalyzed reductive method for the coupling of aryl bromides with secondary alkyl bromides has been developed, providing C(sp2)-C(sp3) products in good to excellent yields. E.g., in presence of NiI2, 4,4'-di-tert-butyl-2,2'-bipyridine, Zn, and MgCl2, reductive coupling of PhBr with secondary alkyl bromide I gave II. Slight modification of this protocol allows efficient coupling of activated aryl chlorides with cyclohexyl bromide and aryl bromides with allylic acetate.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC38Xos1Smtrw%253D&md5=c7e6c4afc9498e1e5fc18243dd6e4618
9.
Molander, G. A.;
Traister, K. M.;
O’Neill, B. J. Org. Chem. 2014,
79,
5771
[
ACS Full Text 
], [
CAS]
9.
Reductive Cross-Coupling of Nonaromatic, Heterocyclic Bromides with Aryl and Heteroaryl Bromides
Molander, Gary A.; Traister, Kaitlin M.; O'Neill, Brian T.
Journal of Organic Chemistry (2014), 79 (12), 5771-5780CODEN: JOCEAH; ISSN:0022-3263. (American Chemical Society)
Reductive cross-coupling allows the direct C-C bond formation between two org. halides without the need for preformation of an organometallic reagent. A method has been developed for the reductive cross-coupling of nonarom., heterocyclic bromides with aryl or heteroaryl bromides. The developed conditions use an air-stable Ni(II) source in the presence of a diamine ligand and a metal reductant to allow late-stage incorporation of satd. heterocyclic rings onto aryl halides in a functional-group tolerant manner. E.g., in presence of NiCl2.(glyme), 1,10-phenanthroline, 4-ethylpyridine, NaBF4, and Mn in MeOH, cross-coupling of 3-bromotetrahydrofuran and 4-BrC6H4COMe gave 54% I.
https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2cXpt1KksbY%253D&md5=5a6e257b972bd763e6e0552c5498bd2c